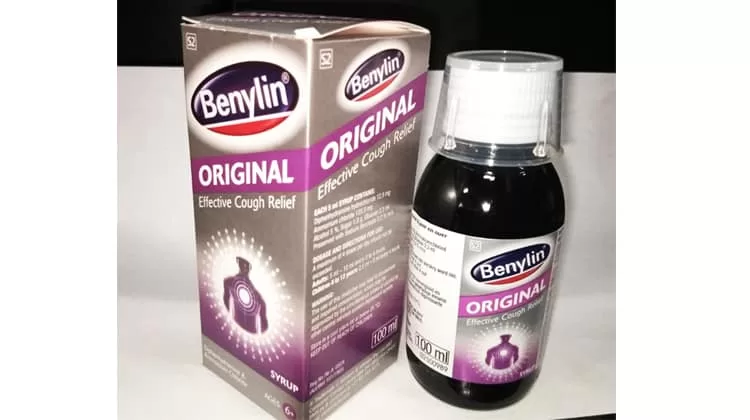
THE Ministry of Health has issued a directive to discontinue the use of Benylin flu medication from two specific batch numbers—329304 and 329303—due to contamination concerns.
health
April 23, 2024
Uncensored News
2 min read
Lesotho bans use of contaminated Benylin batches

Director General of Health Services, Dr, 'Nyane Letsie
Story highlights
Dr. ’Nyane Letsie, the Director General of Health Services at the Ministry, ordered all local health facilities to immediately cease the sale of Benylin from these batches.
"Facilities should remove them from their inventory and return them to the primary importer with immediate effect," stated an alert dated April 15, 2024.
Dr. Letsie also advised that any children exhibiting adverse reactions after using this product should be directed to receive immediate medical attention from a qualified healthcare professional.
Neo Khoarai, a pharmacist with the Ministry of Health, confirmed the validity of the alert and urged calm, noting that only the two batches mentioned are implicated.
The recall follows a similar action by the South African Health Products Regulatory Authority (Sahpra), which recalled two batches of Benylin paediatric cough syrup due to high levels of the toxic chemical diethylene glycol.
A report from the Citizen on April 16 shows that the Nigerian National Agency for Food and Drug Administration and Control also detected high levels of diethylene glycol in a batch of Benylin paediatric cough syrup a week earlier. .
Enjoy our daily newsletter from today
Access exclusive newsletters, along with previews of new media releases.
Sahpra CEO Dr. Boitumelo Semete-Makokotlela warned that the toxic effects of diethylene glycol include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state, and acute kidney injury, which may lead to death.
Dr. Letsie echoed these concerns about the toxicity of diethylene glycol, stressing the substance's lethal potential if consumed.
She also urged consumers or patients who suspect they have encountered substandard or falsified medicines to report to the nearest health facility.
Healthcare professionals are expected to forward these reports to the National Pharmacovigilance Centre. – Uncensored News






